Abstract
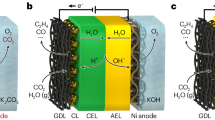
Electrolysis that reduces carbon dioxide (CO2) to useful chemicals can, in principle, contribute to a more sustainable and carbon-neutral future1,2,3,4,5,6. However, it remains challenging to develop this into a robust process because efficient conversion typically requires alkaline conditions in which CO2 precipitates as carbonate, and this limits carbon utilization and the stability of the system7,8,9,10,11,12. Strategies such as physical washing, pulsed operation and the use of dipolar membranes can partially alleviate these problems but do not fully resolve them11,13,14,15. CO2 electrolysis in acid electrolyte, where carbonate does not form, has therefore been explored as an ultimately more workable solution16,17,18. Herein we develop a proton-exchange membrane system that reduces CO2 to formic acid at a catalyst that is derived from waste lead–acid batteries and in which a lattice carbon activation mechanism contributes. When coupling CO2 reduction with hydrogen oxidation, formic acid is produced with over 93% Faradaic efficiency. The system is compatible with start-up/shut-down processes, achieves nearly 91% single-pass conversion efficiency for CO2 at a current density of 600 mA cm−2 and cell voltage of 2.2 V and is shown to operate continuously for more than 5,200 h. We expect that this exceptional performance, enabled by the use of a robust and efficient catalyst, stable three-phase interface and durable membrane, will help advance the development of carbon-neutral technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets that support the findings of this study are presented in the text and Supplementary Information. Source data are provided with this paper.
Change history
14 March 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41586-024-07289-0
References
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Wang, Z. et al. Carbon-confined indium oxides for efficient carbon dioxide reduction in a solid-state electrolyte flow cell. Angew. Chem. Int. Ed. Engl. 61, e202200552 (2022).
Hori, Y. et al. “Deactivation of copper electrode” in electrochemical reduction of CO2. Electrochim. Acta 50, 5354–5369 (2005).
Gao, S. et al. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 529, 68–71 (2016).
Zhu, P. & Wang, H. High-purity and high-concentration liquid fuels through CO2 electroreduction. Nat. Catal. 4, 943–951 (2021).
Xue, W. et al. Operando reconstruction towards stable CuI nanodots with favorable facets for selective CO2 electroreduction to C2H4. Sci. China Chem. 66, 1834–1843 (2023).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Gu, J. et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 5, 268–276 (2022).
Xie, Y. et al. High carbon utilization in CO2 reduction to multicarbon products in acidic media. Nat. Catal. 5, 564–570 (2022).
Ozden, A. et al. Carbon-efficient carbon dioxide electrolysers. Nat. Sustain. 5, 563–573 (2022).
Yang, H., Kaczur, J. J., Sajjad, S. D. & Masel, R. I. Performance and long-term stability of CO2 conversion to formic acid using a three-compartment electrolyzer design. J. CO2 Util. 42, 101349 (2020).
Yan, Z., Hitt, J. L., Zeng, Z., Hickner, M. A. & Mallouk, T. E. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer. Nat. Chem. 13, 33–40 (2021).
Masel, R. I. et al. An industrial perspective on catalysts for low-temperature CO2 electrolysis. Nat. Nanotechnol. 16, 118–128 (2021).
O’Brien, C. P. et al. Single pass CO2 conversion exceeding 85% in the electrosynthesis of multicarbon products via local CO2 regeneration. ACS Energy Lett. 6, 2952–2959 (2021).
Oßkopp, M. et al. Producing formic acid at low pH values by electrochemical CO2 reduction. J. CO2 Util. 56, 101823 (2022).
Li, J. & Kornienko, N. Electrocatalytic carbon dioxide reduction in acid. Chem. Catal. 2, 29–38 (2022).
Bondue, C. J., Graf, M., Goyal, A. & Koper, M. T. M. Suppression of hydrogen evolution in acidic electrolytes by electrochemical CO2 reduction. J. Am. Chem. Soc. 143, 279–285 (2021).
Deng, P. et al. Metal-organic framework-derived carbon nanorods encapsulating bismuth oxides for rapid and selective CO2 electroreduction to formate. Angew. Chem. Int. Ed. Engl. 59, 10807–10813 (2020).
Gao, D. et al. Designing electrolyzers for electrocatalytic CO2 reduction. Acta Phys. Chim. Sin. 37, 2009021 (2021).
Lopes, P. P. & Stamenkovic, V. R. Past, present, and future of lead–acid batteries. Science 369, 923–924 (2020).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Wang, Z. et al. Advanced catalyst design and reactor configuration upgrade in electrochemical carbon dioxide conversion. Adv. Mater. https://doi.org/10.1002/adma.202303052 (2023).
Endrodi, B. et al. Operando cathode activation with alkali metal cations for high current density operation of water-fed zero-gap carbon dioxide electrolysers. Nat. Energy 6, 439–448 (2021).
Wu, Y. et al. Mitigating electrolyte flooding for electrochemical CO2 reduction via infiltration of hydrophobic particles in a gas diffusion layer. ACS Energy Lett. 7, 2884–2892 (2022).
Xing, Z., Hu, L., Ripatti, D. S., Hu, X. & Feng, X. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment. Nat. Commun. 12, 136 (2021).
Overa, S. et al. Enhancing acetate selectivity by coupling anodic oxidation to carbon monoxide electroreduction. Nat. Catal. 5, 738–745 (2022).
Xiao, F. et al. Atomically dispersed Pt and Fe sites and Pt–Fe nanoparticles for durable proton exchange membrane fuel cells. Nat. Catal. 5, 503–512 (2022).
Zhang, Z. et al. Conversion of reactive carbon solutions into CO at low voltage and high carbon efficiency. ACS Cent. Sci. 8, 749–755 (2022).
Fan, L., Xia, C., Zhu, P., Lu, Y. & Wang, H. Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor. Nat. Commun. 11, 3633 (2020).
Jouny, M., Luc, W. & Jiao, F. General techno-economic analysis of CO2 electrolysis systems. Ind. Eng. Chem. Res. 57, 2165–2177 (2018).
Shin, H., Hansen, K. U. & Jiao, F. Techno-economic assessment of low-temperature carbon dioxide electrolysis. Nat. Sustain. 4, 911–919 (2021).
Shi, Y. et al. Unveiling hydrocerussite as an electrochemically stable active phase for efficient carbon dioxide electroreduction to formate. Nat. Commun. 11, 3415 (2020).
Lee, C. H. & Kanan, M. W. Controlling H+ vs CO2 reduction selectivity on Pb electrodes. ACS Catal. 5, 465–469 (2014).
Chen, Y., Li, C. W. & Kanan, M. W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 134, 19969–19972 (2012).
Zhang, Z. et al. Revealing structural evolution of PbS nanocrystal catalysts in electrochemical CO2 reduction using in situ synchrotron radiation X-ray diffraction. J. Mater. Chem. A 7, 23775–23780 (2019).
Newville, M. IFEFFIT: interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 8, 322–324 (2001).
Lu, Z. et al. High-efficiency oxygen reduction to hydrogen peroxide catalysed by oxidized carbon materials. Nat. Catal. 1, 156–162 (2018).
Verma, S., Kim, B., Jhong, H. R., Ma, S. & Kenis, P. J. A gross-margin model for defining technoeconomic benchmarks in the electroreduction of CO2. ChemSusChem 9, 1972–1979 (2016).
Ramdin, M. et al. Electroreduction of CO2/CO to C2 products: process modeling, downstream separation, system integration, and economic analysis. Ind. Eng. Chem. Res. 60, 17862–17880 (2021).
Sisler, J. et al. Ethylene electrosynthesis: a comparative techno-economic analysis of alkaline vs membrane electrode assembly vs CO2–CO–C2H4 tandems. ACS Energy Lett. 6, 997–1002 (2021).
Jing, X., Li, F. & Wang, Y. Assessing the economic potential of large-scale carbonate-formation-free CO2 electrolysis. Catal. Sci. Technol. 12, 2912–2919 (2022).
Jin, J. et al. Constrained C2 adsorbate orientation enables CO-to-acetate electroreduction. Nature 617, 724–729 (2023).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations*. Phys. Rev. B 13, 5188–5192 (1976).
Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 21, 084204 (2009).
Nørskov, J. K. et al. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 152, J23 (2005).
Acknowledgements
This work is financially supported by National Science Foundation for Distinguished Young Scholars (no. 22325901), the National Key Research and Development Program of China (nos. 2021YFA1600800 and 2021YFA1501000), the National Natural Science Foundation of China (no.22075092), the Program for HUST Academic Frontier Youth Team (nos. 2018QYTD15 and 2019QYTD11), the Huazhong University of Science and Technology–Queen Mary University of London Strategic Partnership Research Funding (no. 2022-HUST-QMUL-SPRF-03) and the Innovation and Talent Recruitment Base of New Energy Chemistry and Device (no. B21003). We thank beamline BL01B (for finfrared spectroscopy and microspectroscopy) and BL12B X-ray magnetic circular dichroism (XMCD) at NSRL and beamline 1W1B (XAFS) at BSRF. We thank BL20U and BL14W1 at the Shanghai Synchrotron Radiation Facility for providing the beam time. The computational study is supported by Marsden Fund Council from Government funding (no. 21-UOA-237) and a Catalyst: Seeding General Grant (no. 22-UOA-031-CGS), managed by Royal Society Te Apārangi. Z.W. and R.L. acknowledge the use of New Zealand eScience Infrastructure high-performance computing facilities, consulting support and/or training services as part of this research. We acknowledge the support of WNLO of HUST and the Analytical and Testing Center of Huazhong University of Science and Technology for XRD, XPS, Raman, SEM, TEM, FTIR, inductively coupled plasma–mass spectrometry and NMR measurements. We also thank L. Zhuang at Wuhan University, J. L. Gong at Tianjin University, D. F. Gao at the Dalian Institute of Chemical Physics and J. J. Ge at the University of Science and Technology of China for fruitful discussions.
Author information
Authors and Affiliations
Contributions
B.Y.X. conceived and supervised the project. W.F. and B.Y.X. designed and carried out electrochemical experiments. R.L. carried out DFT calculations. Z.W. and B.Y.X. supervised and advised on DFT calculations. W.F., X.L., S.W., F.S., T.Y. and B.Y.X. performed and discussed soft X-ray absorption spectroscopy characterization. W.F., D.W., X.Y. and T.Y. assisted with in situ FTIR experiments. C.H. assisted with ultraviolet experiments. W.F., Y.L. and T.Z. helped with the in situ Raman test. W.G., F.M.L., C.X., Y.Y. and H.N. contributed to results discussion and data analysis. Y.Z. contributed to XPS result discussion. L.D. provided in situ XRD measurements. W.G., Y.M., C.Z., Y.P., G.W., X.G., B.Y. and B.T. commented on and revised the manuscript. W.F., R.L., T.Y., Z.W. and B.Y.X. wrote and revised the manuscript. All authors discussed the results and assisted with manuscript preparation. W.F., W.G. and R.L. contributed equally to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Feng Jiao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file includes techno-economic analysis (comprehensive TEA calculation procedures and outcomes), Supplementary Figs. 1–47 and Tables 1–7 (comparative analysis of CO2 electrolysis performance, TEA parameters, ion chromatography and DFT results).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, W., Guo, W., Lu, R. et al. Durable CO2 conversion in the proton-exchange membrane system. Nature 626, 86–91 (2024). https://doi.org/10.1038/s41586-023-06917-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06917-5